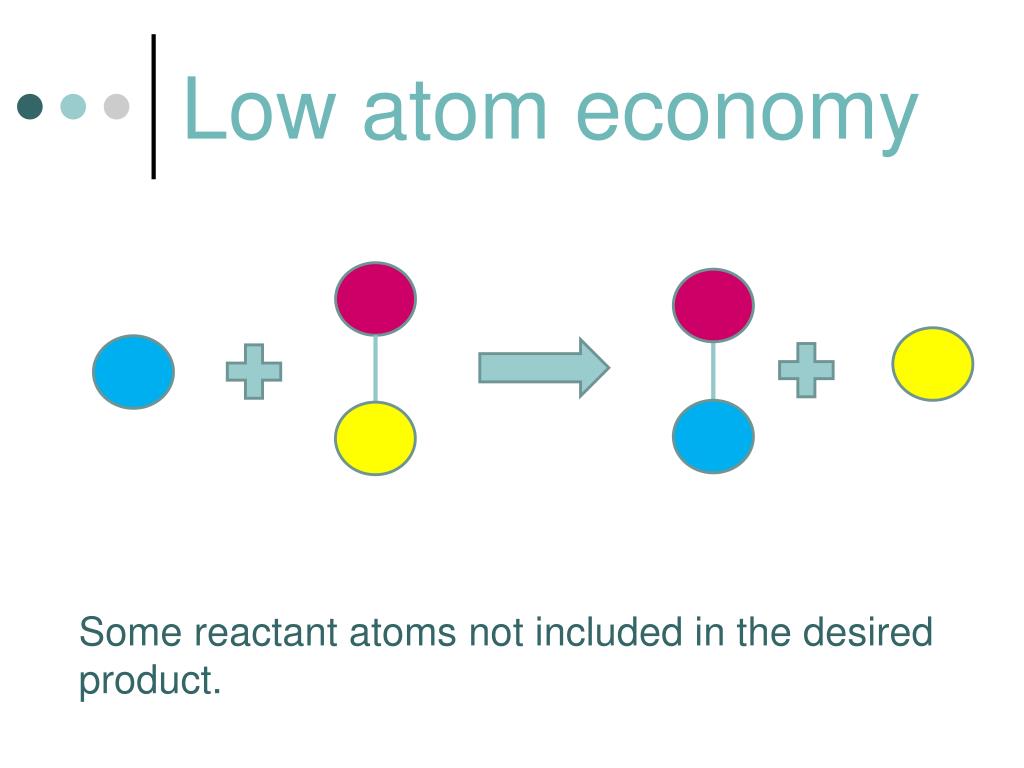
The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Inefficient, wasteful processes have low atom economies. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.
The atom economy of a reaction is a measure of the amount of starting materials that end up as useful products. It is important for sustainable development and for economic reasons to use reactions. % atom economy = (mass of desired product / total mass of products) x 100. Remember this formula in your a level chemistry revision to ensure your grade! The higher the answer, the more efficient the process is (as long as the reaction goes to completion). A reaction which has only one product has an atom economy of 100%.
Companies want to maximise as much product as possible to allow for maximum profits. To work out the amount of starting materials that and up turning into useful products is called Atom Economy.
Atom Economy Example
% of atom economy = Mr of useful products x100
Total Mr of all products
e.g. C2H5OH -> C2H4 + H2O
Mr: C2H5OH = (12×2) +(1×6) + 16 = 46
C2H4 = (12×2) + (1×4) = 28
H2O = (1×2) + 16 = 18

Atom Economy = 28 x100 = 61%
(28+18)
When a chemical reaction is carried out to create a product ,other products are usually made as well. These are called by-products.
The percentage of the total mass of reactants that can theoretically be converted into the product desired is known as the atom economy of a reaction. This can be calculated using the following equation:
% atom economy = (mass of desired product / total mass of products) x 100
Remember this formula in your a level chemistry revision to ensure your grade!
The higher the answer, the more efficient the process is (as long as the reaction goes to completion).
A reaction which has only one product has an atom economy of 100%.
Calculating the atom economy can be useful for figuring out the best process by which to make a particular product.
——————————————————
Reacting Masses
The masses of reactants and products that will react with each other can be calculated using the idea that the number of moles is equal to the mass.
There are two main factors that must be taken into consideration when carrying out calculations that include reacting masses:
Atom Economy Is Quizlet
- The law of conservation of mass: the total combined mass of the reactants must equal the total combined mass of the products.
- The ratio in which the species react has to correspond to the number of moles rather than their mass. This means that masses have to be converted into moles in order to compare each other.
A lot of inorganic reactions go to completion. These reactions are called quantitative due to the fact that they can be analysed in this way.
Other reactions, in particular organic reactions, do not go to completion. The product percentage yield can be calculated using the following equation:

% yield = (amount of product formed / maximum amount of product possible) x 100
——————————————————
The Mole
Due to the that fact atoms are so small all substances contain a lot of them. It is not easy to work with such large numbers and so scientists use moles instead.
A mole of a substance is the amount of that substance that contains the same number of elementary particles as there are carbon atoms in 12.00000 grams of carbon-12.
The number of particles in one mole of a substance is 6.02 x 1023. This is called Avogadro’s number (L).
In order to figure out the number of particles that make up a substance it is easier to count the moles than the particles themselves. The number of particles can than be calculated using Avogadro’s number.
number of particles = number of moles x L
The molar mass of a substance is the mass of one mole. It is measured in gmol-1.
The symbol used for the molar mass of compounds or molecular elements is mrwhile the symbol used for the molar mass of an atom is ar.
Mass (m) which is measured in grams, molar mass (mr or ar) which is measured in gmol-1, and the number of moles (n) are related through the following equation:
Atom Economy Definition
m = mr x n
